25 Degrees C In Kelvin
marihuanalabs
Sep 09, 2025 · 6 min read
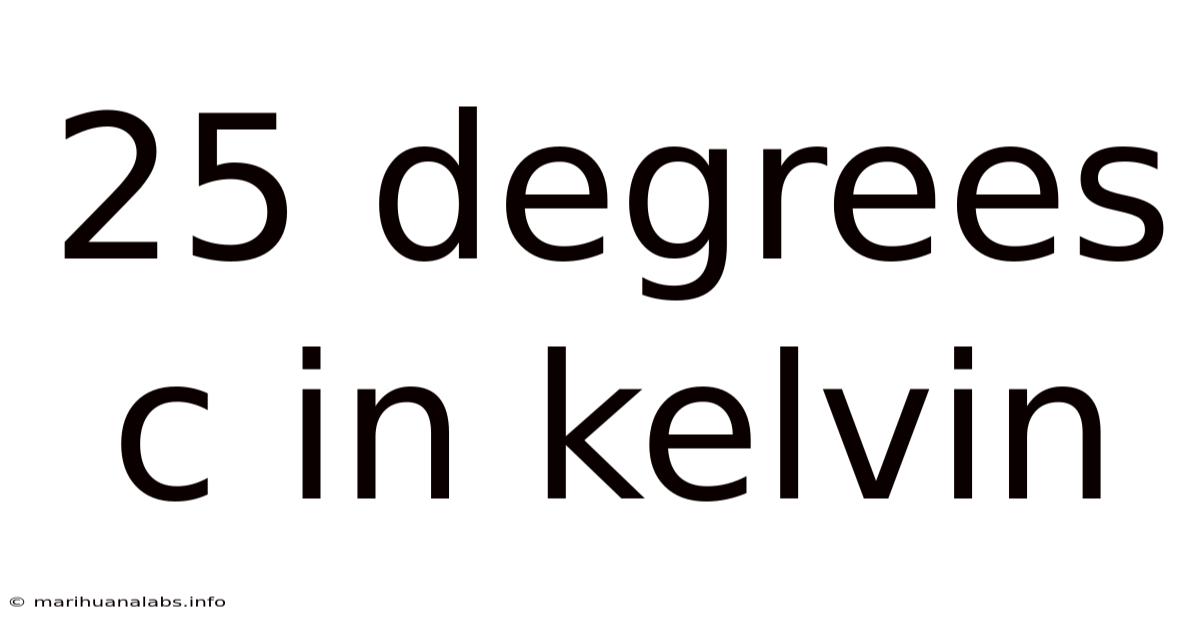
Table of Contents
25 Degrees Celsius in Kelvin: A Comprehensive Guide to Temperature Conversion
Understanding temperature scales is crucial in various fields, from everyday life to advanced scientific research. While Celsius (°C) is widely used for everyday temperature measurements, Kelvin (K) is the absolute temperature scale used extensively in scientific calculations and engineering. This article provides a comprehensive guide to converting 25 degrees Celsius to Kelvin, explaining the process, the underlying scientific principles, and its implications in different contexts. We'll delve into the history of these scales, explore practical applications, and answer frequently asked questions to solidify your understanding of this essential temperature conversion.
Understanding Temperature Scales: Celsius and Kelvin
Before we delve into the conversion, let's briefly review the two temperature scales involved: Celsius and Kelvin.
-
Celsius (°C): This scale, also known as the centigrade scale, is based on the freezing and boiling points of water at standard atmospheric pressure. Water freezes at 0°C and boils at 100°C. This scale is commonly used for everyday temperature measurements globally.
-
Kelvin (K): This is the absolute temperature scale, meaning it starts at absolute zero – the theoretical point where all molecular motion ceases. Absolute zero is defined as 0 K, which is equivalent to -273.15°C. Kelvin does not use the degree symbol (°). The size of a Kelvin degree is the same as a Celsius degree.
The key difference between Celsius and Kelvin lies in their zero points. Celsius is relative to the properties of water, while Kelvin is rooted in the fundamental concept of absolute zero.
Converting 25 Degrees Celsius to Kelvin: The Simple Formula
The conversion from Celsius to Kelvin is straightforward and involves a simple addition:
K = °C + 273.15
Therefore, to convert 25°C to Kelvin:
K = 25°C + 273.15 = 298.15 K
So, 25 degrees Celsius is equal to 298.15 Kelvin.
The Scientific Basis of Absolute Zero and the Kelvin Scale
The concept of absolute zero is fundamental to understanding the Kelvin scale. At absolute zero, all matter theoretically possesses zero thermal energy. This means the atoms and molecules making up the substance have minimal kinetic energy; they are essentially stationary. It's important to note that absolute zero is a theoretical limit; it's impossible to actually reach it in practice.
The Kelvin scale is based on the thermodynamic properties of matter, particularly the relationship between temperature and energy. It’s preferred in scientific applications because many physical laws and equations are expressed more simply and elegantly using the Kelvin scale. For example, the ideal gas law, a fundamental equation in chemistry and physics, is best expressed using Kelvin temperature.
Practical Applications of Kelvin Temperature
The Kelvin scale has widespread applications in various scientific and engineering disciplines:
-
Physics: Kelvin is essential in thermodynamics, statistical mechanics, and quantum mechanics. Many physical laws and constants are defined in terms of Kelvin.
-
Chemistry: Chemical reactions and equilibrium calculations often require the use of Kelvin temperatures. This is because the rate and extent of chemical reactions are directly influenced by temperature.
-
Engineering: Engineers working with processes involving heat transfer, thermal expansion, and fluid mechanics typically use the Kelvin scale. For example, designing efficient engines or cooling systems often requires precise temperature calculations in Kelvin.
-
Astronomy: In astrophysics, Kelvin is used to measure the surface temperatures of stars and other celestial bodies. These incredibly high temperatures are best expressed using the absolute temperature scale.
-
Material Science: The behavior of materials, especially at extreme temperatures, is often described and studied using Kelvin. For example, the properties of superconductors are heavily influenced by temperature and are usually characterized using Kelvin.
Understanding the Implications of Using Kelvin
The use of Kelvin instead of Celsius in scientific calculations is not merely a matter of convenience. It leads to several critical advantages:
-
Avoids negative values: The elimination of negative temperatures eliminates potential ambiguity and simplifies many equations.
-
Direct proportionality: In many thermodynamic relationships, a direct proportionality exists between temperature and other properties (e.g., the volume of an ideal gas). Using Kelvin ensures this relationship is linear and simplifies the calculations.
-
Consistent units: Kelvin consistently aligns with other fundamental physical units, simplifying calculations and ensuring dimensional consistency in scientific formulas.
Beyond the Conversion: Deeper Dive into Thermodynamics
Understanding the conversion from Celsius to Kelvin is just the first step in grasping the broader concept of temperature and its significance in thermodynamics. Thermodynamics deals with the relationship between heat, work, and energy in systems. Several key concepts within thermodynamics are directly linked to temperature, and Kelvin plays a central role:
-
Internal Energy: The total energy within a system is directly related to its temperature, particularly when expressed in Kelvin.
-
Entropy: A measure of disorder or randomness in a system, entropy is directly related to temperature. Changes in entropy are often calculated using Kelvin.
-
Heat Capacity: The amount of heat required to raise the temperature of a substance by a specific amount. This value is often determined and expressed using Kelvin.
-
Thermodynamic Equilibrium: A state where there is no net flow of energy or matter between parts of a system. This state is characterized by a uniform temperature, usually expressed in Kelvin.
Frequently Asked Questions (FAQ)
Q1: Why is the Kelvin scale important in science?
A1: The Kelvin scale is crucial in science because it's an absolute scale, starting at absolute zero. This eliminates negative values and simplifies many scientific equations and laws, particularly those in thermodynamics and statistical mechanics. It provides a more fundamental and consistent basis for measuring temperature.
Q2: Can I use Celsius for all scientific calculations?
A2: While Celsius is widely used in everyday life, using Celsius in many scientific calculations could lead to inaccuracies and complexities due to the arbitrary zero point. Kelvin provides a more accurate and consistent basis for scientific calculations, especially in areas involving heat, energy, and thermodynamic properties.
Q3: What happens at absolute zero?
A3: At absolute zero (0 K), all molecular motion theoretically ceases. However, reaching absolute zero is practically impossible due to the laws of quantum mechanics. It remains a theoretical limit.
Q4: Are there other temperature scales besides Celsius and Kelvin?
A4: Yes, the Fahrenheit scale is another commonly used temperature scale, primarily in the United States. However, for scientific applications, the Kelvin scale is the universally accepted standard.
Conclusion: Mastering Temperature Conversions for Scientific Success
Converting 25 degrees Celsius to Kelvin (298.15 K) is a simple mathematical operation, but understanding the underlying principles is crucial for anyone working with scientific or engineering concepts. The Kelvin scale’s foundation in absolute zero and its implications for various scientific laws and equations cannot be overstated. Mastering this conversion not only enhances your understanding of temperature scales but also provides a solid foundation for further exploration of thermodynamics and its diverse applications in various scientific fields. This knowledge allows for accurate calculations, meaningful interpretations of data, and a deeper appreciation for the fundamental principles governing the physical world.
Latest Posts
Latest Posts
-
What Is A Static Pressure
Sep 09, 2025
-
What Is 80g In Ml
Sep 09, 2025
-
Opposite Of The Word Always
Sep 09, 2025
-
Net Weight And Gross Weight
Sep 09, 2025
-
Convert 35 Cm Into Inches
Sep 09, 2025
Related Post
Thank you for visiting our website which covers about 25 Degrees C In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.